April 1st, 2011 at 3:08 pm (Chemistry, History)
As you may have learned in high school chemistry, a mole is 6.022 x 1023 of something (usually something really tiny, like atoms). This seemingly arbitrary value is also known as Avogadro’s number. Why is it named after him, and how did that value come to be chosen?
Avogradro’s full name was Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerret, and he had nothing to do with defining the value for a mole. Instead, he came up with the ideal gas law, which says that two samples of gas that occupy the same volume (with the same temperature and pressure) also contain the same number of molecules. It is most often encountered as pV = nRT. In 1909, 53 years after Avogadro died, Jean Perrin proposed naming the value of a mole after him.
Beyond Avogadro, why use 6.022 x 1023 as the basis for a mole? The history of this decision is an interesting illustration of what happens when a common value must be chosen for convenience, but there is no physical reason to prefer one over another (a similar situation arises for planetary coordinates; latitude has a well defined start point due to the axis of rotation, but longitude has to be given a start point arbitrarily). Originally, and quite logically, chemists used hydrogen (the simplest element, with only one proton) as a basis for computing atomic weights. They set H=1 and computed values for everything else relative to hydrogen. Later others proposed using oxygen as a standard (O=16), since oxygen is so prevalent and combines with so many things, and after decades of debate, in 1961 they finally settled on using carbon (C=12) as the standard. And so the value for a mole was born: the number of atoms in 12 grams of carbon-12 (isotopes matter!). (How do you count them though? See here.)
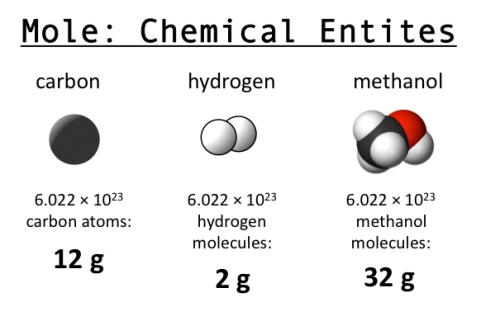 Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)
Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)
Even though it came from just 12 grams of carbon, a mole is a really, really big number. No, really. I love these analogies (from CDLI’s Chemistry 2202 course) that help us understand how big:
How big is a mole?
- one mole of peas is enough to cover Earth and 250 more planets the same size as Earth one metre deep in green peas
- a stack of one mole of pennies is tall enough to reach Proxima Centauri (the second closest star to Earth) and back again 7448 times
- a mole of marbles spread over the Earth would cover it to a depth of 80 km
- if you own a mole of dollars and you spend a billion dollars a day, then you could spend that amount per day for over a trillion years before you run out of money. (Earth has only been around for 4.5 billion years i.e. that’s 0.45% of a trillion years!)
For more molar fun, I highly recommend this mole lesson/activity. And six months from now, you can celebrate Mole Day: October 23, from 6:02 a.m. to 6:02 p.m.
1 Comments
0 of 1 people learned something from this entry.
January 29th, 2011 at 4:34 pm (Chemistry, History)
 It happened so long ago that it’s not really known how glass was first discovered. Certainly, lightning can strike a sandy beach and fuse silicon into glass bits (called fulgurites). The lightning has to reach 3270 F for this to happen, which would be difficult for ancient human cultures to reproduce, although there are tales of sailors inadvertently using sodium carbonate blocks around a fire ring on the beach, which reduces the melting point of glass to 2552 F, more likely to be within reach. According to wikipedia, a candle flame reaches about 1800 F, and a blowtorch gets up to 2370 F, and a Bunsen burner can get up to 2900 F — so chemistry lab students out there should be able to get right to work on glass-making!
It happened so long ago that it’s not really known how glass was first discovered. Certainly, lightning can strike a sandy beach and fuse silicon into glass bits (called fulgurites). The lightning has to reach 3270 F for this to happen, which would be difficult for ancient human cultures to reproduce, although there are tales of sailors inadvertently using sodium carbonate blocks around a fire ring on the beach, which reduces the melting point of glass to 2552 F, more likely to be within reach. According to wikipedia, a candle flame reaches about 1800 F, and a blowtorch gets up to 2370 F, and a Bunsen burner can get up to 2900 F — so chemistry lab students out there should be able to get right to work on glass-making!
 Interestingly, glass made with sodium carbonate (also called niter) isn’t very stable; it gradually dissolves from contact with water (which makes terrible windows or water glasses). More stable glass can be made by adding calcium carbonate (lime), which is what is still done today. Other additives create color tints and other properties. Here are some glass recipes with suggested proportions. The wikipedia article on glass is full of other fascinating tidbits.
Interestingly, glass made with sodium carbonate (also called niter) isn’t very stable; it gradually dissolves from contact with water (which makes terrible windows or water glasses). More stable glass can be made by adding calcium carbonate (lime), which is what is still done today. Other additives create color tints and other properties. Here are some glass recipes with suggested proportions. The wikipedia article on glass is full of other fascinating tidbits.
And yes, glass is generally considered to be a solid (albeit an amorphous one, meaning it has no crystalline structure) rather than a liquid.
1 Comments
0 of 1 people learned something from this entry.
January 17th, 2011 at 8:56 am (Art, Engineering, History)
High-speed photography can capture athletes in action. But you need really high-speed photography to capture events like a nuclear explosion.
At the recent National Radio Science Meeting, I first encountered the idea of a rapatronic camera. These cameras have exposure times as short as 10 ns. They were developed in 1940 to capture the rapid expansion of a nuclear explosion, and they were gems of ingenuity. No mechanical shutter at that time could possibly open and close that quickly, so Harold Edgerton came up with a non-mechanical way of controlling the shutter: he put a Kerr cell between two polarizing filters oriented at 90 degrees from each other. Normally, no light would penetrate between the crossed polars. But when voltage is applied to the Kerr cell, it rotates the polarization of the incoming light 90 degrees—permitting it to pass through the second filter. By only activating the Kerr cell for a very short time, you obtain an ultra high-speed shutter.
You can view Edgerton’s hand-drawn circuit diagram to see how it worked.
Likewise, there was no way to mechanically advance the film fast enough to permit a single camera to take a sequence of high-speed shots, so in these tests they’d set up an array of the cameras, each with a slightly different delay. (Although I immediately wonder if you couldn’t have an electronically controlled refractive material behind the single lens to direct the light across a series of film segments so you wouldn’t have to physically move anything.)
The results are stunning:


At left is an explosion from Operation Tumbler-Snapper (1952), about 1 ms after detonation. The spikes along the bottom edge are evidence of the tower’s guy wires being vaporized by associated gamma rays. At right is an explosion from Operation Hardtack II (1958). This one was suspended from a balloon and the spikes here are the balloon’s mooring cables being vaporized. A beautiful ghostly array of such images is available from a
google image search on “rapatronic”.
One thing I haven’t been able to determine is the etymology of “rapatronic”. It may be that Edgerton just coined it (with “rapa” for “rapid” and “tronic” for “electronic”—but that’s just a guess). Please share if anyone knows more!
You can read more about Edgerton and his various innovations aside from the rapatronic camera. Brilliant guy!
4 Comments
4 of 4 people learned something from this entry.
November 18th, 2010 at 8:43 pm (History, Literature)
 I recently discovered audiobook versions of The Adventures of Sherlock Holmes, through the lit2go project, via iTunes University. I was inspired to download these stories by my recent reading of “A Study in Scarlet”, and now I’m wondering how I ever missed out on the whole series as a kid! Or even as an adult! These stories are great fun to read, written in the most marvelous language (and, through lit2go, shared by an excellent British-accented reader).
I recently discovered audiobook versions of The Adventures of Sherlock Holmes, through the lit2go project, via iTunes University. I was inspired to download these stories by my recent reading of “A Study in Scarlet”, and now I’m wondering how I ever missed out on the whole series as a kid! Or even as an adult! These stories are great fun to read, written in the most marvelous language (and, through lit2go, shared by an excellent British-accented reader).
The first story in this collection, “A Scandal in Bohemia,” tells of one of the very few times Holmes failed at his game… and in this case, he was bested by a woman. Read this opening paragraph, and I challenge you not to read the whole thing:
To Sherlock Holmes she is always THE woman. I have seldom heard him mention her under any other name. In his eyes she eclipses and predominates the whole of her sex. It was not that he felt any emotion akin to love for Irene Adler. All emotions, and that one particularly, were abhorrent to his cold, precise but admirably balanced mind. He was, I take it, the most perfect reasoning and observing machine that the world has seen, but as a lover he would have placed himself in a false position. He never spoke of the softer passions, save with a gibe and a sneer. They were admirable things for the observer—excellent for drawing the veil from men’s motives and actions. But for the trained reasoner to admit such intrusions into his own delicate and finely adjusted temperament was to introduce a distracting factor which might throw a doubt upon all his mental results. Grit in a sensitive instrument, or a crack in one of his own high-power lenses, would not be more disturbing than a strong emotion in a nature such as his. And yet there was but one woman to him, and that woman was the late Irene Adler, of dubious and questionable memory.
From there we have a sequence of excellent mysteries, my favorite so far being “The Five Orange Pips.” I didn’t know that the Ku Klux Klan took its name from the distinctive sound of a rifle being cocked—although this may be a fanciful myth (wikipedia instead claims that “The name was formed by combining the Greek kyklos (κυκλος, circle) with clan”). I also didn’t know that they were in the habit of sending dried orange bits as a dreadful warning to their next victims. According to Sir Arthur Conan Doyle:
Its [the KKK’s] outrages were usually preceded by a warning sent to the marked man in some fantastic but generally recognized shape—a sprig of oak-leaves in some parts, melon seeds or orange pips in others. On receiving this the victim might either openly abjure his former ways, or might fly from the country. If he braved the matter out, death would unfailingly come upon him, and usually in some strange and unforeseen manner.
True or not (and this is of course fiction), it certainly creates a powerful image!
I’m now on to “The Adventure of the Blue Carbuncle,” eager to find out how the mysterious blue stone could have gotten into the craw of a Christmas goose. Keep up the good work, Holmes and amanuensis Watson!
Comments
November 4th, 2010 at 10:53 pm (History, Literature)
He was a man of independence and learning, with passion for his convictions and love for nature. But less often do we hear of Thoreau’s feelings towards women. His biographers note his marriage proposal to Ellen Seawall (who rejected him) and the love poems he sent to Mary Russell (whom he never proposed to). More mysteriously, Thoreau himself wrote in his diary:
“The obstacles which the heart meets with are like granite blocks which one alone cannot move. She who was as the morning light to me is now neither the morning star nor the evening star. We meet but to find each other further asunder, and the oftener we meet the more rapid our divergence. So a star of the first magnitude pales in the heavens, not from any fault in the observer’s eye nor from any fault in itself, perchance, but because its progress in its own system has put a greater distance between.”
This was however recorded 10 years after his compositions for Mary. Was he looking back on the past? Or was he enamored of another, whose ardor for him had dimmed? No matter the reason, this poignant prose speaks of a loss and melancholy that reaches across the years to today.
[Thanks to The Blog of Henry David Thoreau, which posts excerpts from his diaries on a daily basis.]
Robert Frost had some similar sentiments to share:
Ah, when to the heart of man
Was it ever less than a treason
To go with the drift of things,
To yield with a grace to reason,
And bow and accept the end
Of a love or a season?
— Reluctance
This treason of acceptance is, however painful, a part of life. But our poets can take that moment and paint it in beautiful words and hang it on the wall for us to acknowledge, and consider, and deliberate upon. Who does not prefer, in theory, to yield with grace to reason? And yet who does not find it, in reality, to be all-nigh unbearable?
2 Comments
1 of 1 people learned something from this entry.
 Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)
Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)



